Question #1
A 4-year-old boy is brought to the physician because of a large, painful boil on his left knee. He has a history of repeated infections with Candida, Staphylococcus, and Klebsiella since the age of 14 months. Laboratory studies show an abnormal result from the nitroblue tetrazolium reduction test. Cultures of the fluid from the boil grow Staphylococcus aureus.
Which of the following intracellular defense mechanisms is most likely absent in this patient?
Defensins
Hydrolytic enzymes
Lysozymes
Myeloperoxidase
NADPH oxidase
Answer:
The correct answer is E.
This child has chronic granulomatous disease (CGD), which is a genetic defect in one or more of the subunits of NADPH oxidase. Activation of this membrane-bound oxidase is the critical first step in the generation of toxic oxygen radicals within the phagolysosome. One of the products of this reaction, hydrogen peroxide, serves as the substrate for the second most important killing mechanism: myeloperoxidase acting to produce toxic halide radicals. Patients with CGD are prone to persistent infections with catalase-positive bacteria and fungi, because these organisms destroy the hydrogen peroxide that they produce metabolically, so neither of the oxygen-dependent mechanisms can function in intracellular killing during these infections. Typical catalase-positive microbes include Staphylococcus, Klebsiella, Serratia, and Aspergillus.
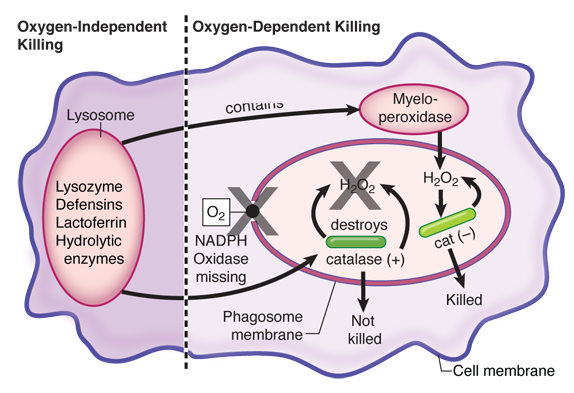
Failure of phagocytic cells to generate oxygen radicals are easily detected by the nitroblue tetrazolium (NBT) reduction test (see below).
Defensins (choice A), hydrolytic enzymes (choice B), and lysozymes (choice C) are part of the oxygen-independent killing mechanisms of phagocytic cells. These make up the least powerful of the intracellular killing mechanisms used by phagocytes, and there are no known disease states that result from such deficiencies.
Myeloperoxidase (choice D) is an enzyme present in lysosomes that acts on hydrogen peroxide to produce toxic halide radicals such as hypochlorite.
Question #2
A 52-year-old woman comes to the physician for a routine examination. She has been experiencing periods of heat intolerance, which she attributes to menopause. Physical examination shows unusually protuberant eyeballs and sinus tachycardia. Laboratory studies show a serum T3 of 5.3 nmol/L and a T4 of 225 nmol/L.
Which of the following hypersensitivities is the most likely mechanism of pathogenesis of her condition?
Type I
Type II cytotoxic
Type II noncytotoxic
Type III
Type IV
Answer:
The correct answer is C.
This patient has Graves disease, an autoimmune form of hyperthyroidism produced by autoantibodies directed against the thyroid-stimulating hormone (TSH) receptor. These antibodies are called long-acting thyroid stimulator (LATS) and they stimulate thyroid function, resulting in the release of thyroid hormones. The exophthalmia associated with this disease is due to edema of the extraocular muscles. This is a type II non-cytotoxic hypersensitivity in which antibodies occupy the TSH receptor sites and provide unrelenting stimulation to the thyroid cells, which ultimately become exhausted and unable to further secrete T3 and T4. It is distinctive from other forms of type II hypersensitivity in that the pathology does not involve complement-mediated lysis of the affected cells. It is distinguished from Hashimoto thyroiditis (a type IV hypersensitivity) because biopsy of the Graves disease thyroid would not show evidence of cell-mediated cytolysis.
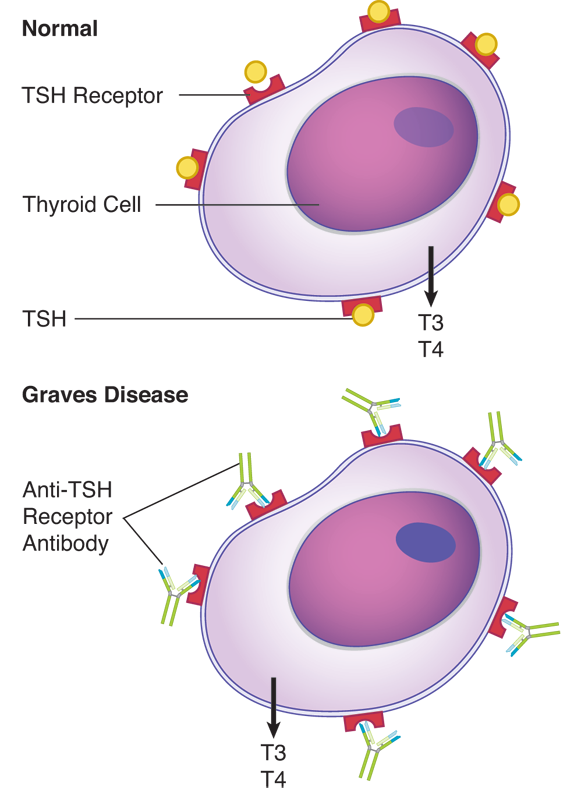
Type I hypersensitivity (choice A) is also known as IgE-mediated hypersensitivity, immediate hypersensitivity, or atopic allergy. Its symptoms manifest within minutes of reintroduction of an allergen, and are caused by cross-linkage of IgE molecules bound to mast cells and basophils and the resulting degranulation of those cells causing tissue damage.
Type II cytotoxic hypersensitivity (choice B) involves autoantibodies, which bind to specific tissues or cells, activate complement, and cause the destruction of the underlying tissue.
Type III hypersensitivity (choice D) is also known as immune complex hypersensitivity. It is caused by the deposition of complexes of antigen and antibody, causing complement activation in the small vasculature where the complexes are cleared from the circulation. The damage is mediated by complement activation, and is system-wide rather than organ-specific.
Type IV hypersensitivity (choice E) is also known as T cell-mediated or delayed-type hypersensitivity. It is mediated by TH1 cells and macrophages and is manifested 48 to 72 hours after reintroduction of protein antigens. Hashimoto thyroiditis is an example of this type of hypersensitivity and is diagnosed by the finding of cell-mediated cytolysis in a biopsy of the thyroid.
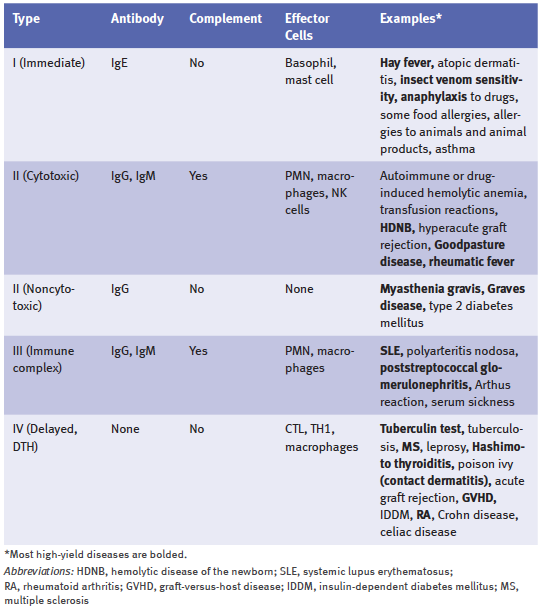
The table below shows the classification of immunologic diseases.
The table below shows a summary of hypersensitivity reactions.
Question #3
A study is conducted to evaluate the effect of cytokine administration on the progression of multi-drug resistant tuberculosis in HIV-positive patients. A variety of cytokines produced by recombinant DNA are aerosolized and administered twice daily to a group of 20 individuals. Treatment progress is monitored by chest x-ray and sputum culture.
Which of the following cytokines is likely to have the most beneficial effect on macrophage intracellular killing of the mycobacteria?
Interferon-alpha
Interferon-beta
Interferon-gamma
Interleukin-2
Interleukin-6
Answer:
The correct answer is C.
Mycobacterium tuberculosis is an intracellular pathogen that lives inside macrophages. The protective immune response to this organism depends on the stimulation of more efficient intracellular killing in phagocytic cells by the cytokines of T helper-1 (TH1) cells. Interferon (IFN)-gamma is a product of TH1 cells that acts on macrophages to enhance their microbicidal activities. IFN-gamma is one of the most potent activators or macrophages.
The figure below provides an overview of cell-mediated immunity.
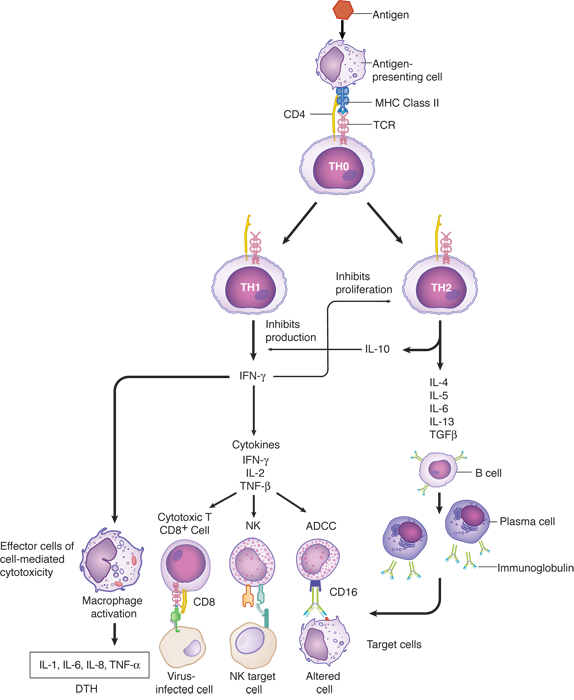
IFN-alpha (choice A) is a product of leukocytes that inhibits viral replication. It is unlikely to have an effect on an intracellular bacterial infection.
IFN-beta (choice B) is a product of fibroblasts that inhibits viral replication. It is unlikely to have an effect on an intracellular bacterial infection.
Interleukin (IL)-2 (choice D) is a product of TH cells that causes proliferation of other lymphocytes, therefore macrophages would not be affected by IL-2 administration.
Interleukin-6 (choice E) is a pluripotent cytokine that is intimately associated with the inflammatory response to injury or infection. The cellular and physiologic effects of IL-6 are diverse and include, but are not limited to, induction of fever and induction of the synthesis of acute-phase proteins (e.g., C-reactive proteins).
Question #4
A 51-year-old man with a history of hepatitis B infection develops fulminant hepatitis. A liver transplant is performed. One year later, he comes to the physician for a follow-up examination. Laboratory studies show AST of 83 U/L, ALT of 102 U/L, and total bilirubin of 3.2 mg/dL. Therapy is begun to minimize chronic rejection.
Immunosuppressive therapy should be directed toward downregulating which of the following components of the immune response?
Autoantibody production
Complement protein synthesis
HLA antigen expression
Mast cell degranulation
T-lymphocyte activity
Answer:
The correct answer is E.
Chronic rejection of any solid organ entails injury to endothelial cells, resulting in intimal proliferation, fibrosis, and eventually ischemic injury to the graft. Immunosuppressive therapy is directed at controlling T-lymphocyte activity. Because T cells regulate the immune response, therapy is directed at killing T cells, suppressing T-cell division, or suppressing T-cell cytokine production. The timeframe to developing chronic rejection is months to years.
To review, hyperacute rejection can occur in minutes to hours and is the result of preformed antibodies binding to the graft cells. Conditions under which these antibodies can be produced include prior transplantation, pregnancy and blood transfusions containing white blood cells. The antibodies are generated against the foreign MHC molecules but crossing the ABO blood group barrier can also cause hyperacute rejection. Acute rejection takes place within a month of the transplant and is the result of normal humoral and/or cell-mediated immune responses against the foreign MHC molecules of the graft.
Autoantibodies (choice A) are not involved in organ transplant rejection. The antibodies produced are alloantibodies directed only against the graft, not the host.
Complement proteins (choice B) are involved in the humoral component of acute rejection, and complement binding to alloantibodies increases graft damage. Complement protein production, however, is not affected by immunosuppressive therapy.
HLA antigen expression (choice C) is central to recognition of foreign cells in grafted tissue. HLA antigens are expressed constitutively by all normal cells, and immunosuppression does not affect their production.
Mast cell degranulation (choice D) is a component of the anaphylactic response (type I hypersensitivity). Chronic graft rejection is a type IV hypersensitivity response and does not involve mast cell degranulation.
Question #5
A 4-month-old boy is brought to the physician by his parents because of twitching of his facial muscles. A review of his records shows that he has previously been seen for several severe episodes of Candida infections. Physical examination shows low-set ears, hypertelorism, and a shortened philtrum.
Which of the following additional findings is most likely to be seen in this patient?
Absence of type IV hypersensitivity
Decreased alpha-fetoprotein
Elevated IgE levels
Elevated IgM levels
Prominent ocular telangiectasias
Answer:
The correct answer is A.
These clinical findings describe DiGeorge syndrome, which is generally caused by a microdeletion (of band 22q11.2). Clinically, patients present with tetany (usually first noted in the facial muscles) due to hypocalcemia secondary to hypoparathyroidism. The thymus is absent, as are the parathyroid glands, due to failure of development of the 3rd and 4th pharyngeal pouches. It should be noted that most DiGeorge syndrome patients are not completely athymic, but rather are partial DiGeorge syndrome patients in that they retain some functional thymic tissue. The immunodeficiency observed in partial DiGeorge syndrome patients tends to be less severe than those who are born with a total absence of a thymus.
Recurrent infections due to defective cellular immunity, and abnormal facies are additional features. Since patients without a thymus are unable to complete normal T-lymphocyte development, these patients will have deficiencies in all immunologic reactions dependent on T cells. Type IV hypersensitivity, which is totally dependent on T lymphocytes and their cytokines, would be absent from these patients. A mnemonic to remember the key features of DiGeorge syndrome is CATCH-22 (Cardiac abnormality, Abnormal facies, Thymic aplasia, Cleft palate, Hypocalcemia, resulting from a deletion on chromosome 22).
Decreased alpha-fetoprotein (choice B) is an amniotic fluid marker for Down syndrome. Decreased levels of estriol and increased levels of hCG and inhibin are also associated with Down syndrome. Down syndrome patients have abnormal immune responses that predispose them to serious infections (particularly of the lungs) and to thyroid autoimmune disease. However, there is no defect of the parathyroid glands or the thymus, and the facial features of Down syndrome are inconsistent with those described here.
Elevated IgE levels (choice C) are found in persons with atopic allergy. Since IgE isotype-switching depends on TH2 cytokines, patients with DiGeorge syndrome should be unable to develop allergy.
Elevated IgM (choice D) is seen in hyper-IgM syndrome. Patients have a high concentration of IgM and normal numbers of T and B cells, but low levels of IgG, IgA, and IgE. Helper T cells have a defect in the surface protein CD40 ligand that interacts with CD40 on the B-cell surface. This results in an inability of the B cell to switch from the production of IgM to other classes of antibodies. In patients with DiGeorge syndrome, the relative amount of IgM could be elevated because they would be unable to promote isotype-switching and their B lymphocytes would therefore be "stuck" producing IgM. It is unlikely, however, that the total IgM concentration in the serum would be significantly altered.
Prominent telangiectasias, which are readily observed as capillary distortions in the conjunctiva of the eyes (can be mistaken for conjunctivitis) (choice E) are seen as part of the ataxia-telangiectasia syndrome, as are gait abnormalities (ataxia), and reduced IgA and IgE production. This is an autosomal recessive disorder, and is also referred to as a chromosomal breakage syndrome. Ataxia-telangiectasia is associated with increased numbers of translocations, especially involving the T-cell receptor loci; the gene for this disorder has been mapped to chromosome 11. Patients have an increased incidence of malignancy.
Now that you've tasted a few questions, are you ready for more?
Enroll in our Step 1 Qbank and practice with over 2,000 USMLE-style questions, now with a lower price. Start now with our Until Your Test® access and get a higher score.
Fill out the information below to get the answers and explanations. We’ll also email you free tips, admissions advice, exclusive offers, and other content aimed specifically to help you do better on Test Day.